Micro Extraction by Packed Sorbent
From LEAP
| Line 233: | Line 233: | ||
Rockaway NJ 07866 | Rockaway NJ 07866 | ||
---- | ---- | ||
| - | |||
[[Image:MEPS.png|center|1000px]]<br><br> | [[Image:MEPS.png|center|1000px]]<br><br> | ||
| - | + | {{logo}} | |
LEAP provides automated workstation instrumentation solutions based on the LEAP CTC PAL X, Y, Z syringe only autosampler robot from LEAP Technologies. This extremely flexible, precise, and adaptable liquid handling robotic platform is available in a variety of lengths and options depending on the requirements of your sample preparation and injections for your UHPLC, LC or GC chromatography.LEAP offers full support and service for the PAL platform in addition to being able to write custom macros, cycles, and scheduling to your applications. Please contact LEAP Technologies on how we can help you get maximized throughput with flexible pipetting automation solutions. | LEAP provides automated workstation instrumentation solutions based on the LEAP CTC PAL X, Y, Z syringe only autosampler robot from LEAP Technologies. This extremely flexible, precise, and adaptable liquid handling robotic platform is available in a variety of lengths and options depending on the requirements of your sample preparation and injections for your UHPLC, LC or GC chromatography.LEAP offers full support and service for the PAL platform in addition to being able to write custom macros, cycles, and scheduling to your applications. Please contact LEAP Technologies on how we can help you get maximized throughput with flexible pipetting automation solutions. | ||
Revision as of 16:33, 4 November 2009

| SPE Applications |
| Application Type | |
| STANDARD AND SPECIAL | |
| Application ID | |
| MEPS | |
| Description | |
| Micro Extraction by Packed Sorbent (MEPS) |
Contents |
Overview
Micro Extraction by Packed Sorbent* (MEPS) is a new development in the field of sample preparation by solid phase extraction (SPE), and is a result of a collaboration between SGE and Mohamed Abdel-Rehim (AstraZeneca) and Lars G. Blomberg (University of Karlstead) Automation of MEPS is accomplished with the PAL Series of CTC Autosamplers. MEPS is the miniaturization of conventional SPE packed bed devices from milliliter bed volumes to microliter volumes. The MEPS approach to sample preparation is suitable for reversed phases, normal phases, mixed mode or ion exchange chemistries. MEPS is available in a variety of common SPE phases. The cartridge (Patent Pending) contains the stationary phase, and is built into the syringe needle. With a typical void volume of a few μL, the MEPS elution is compatible with GC and LC inlets making it ideal for automated on-line SPE on the CTC PAL platform. MEPS kits are exclusively available worldwide through LEAP Technologies and SGE Analytical Science.
MEPS performs the same function as SPE, namely the purification or speciation of samples, but with some significant differences:
- MEPS works with much smaller samples (as small as 10µL) than full scale SPE
- MEPS can be fully automated – the sample processing, extraction and injection steps are performed on-line using the same syringe
- MEPS is applicable to GC and LC
- Significantly reduces the volume of solvents and sample needed
MEPS online is an SPE solution for rapid development, in a variety of matrices; plasma, serum, whole blood, urine, aqueous
MEPS for PAL Systems is distributed by SGE & LEAP Technologies.
Benefits of MEPS
- MEPS allows SPE methodology to be applied to small sample volumes.
- MEPS can be integrated into autosampler robotics and allows on-line use of SPE.
- MEPS can reduce sample and reagent consumption and waste disposal.
- Double pass flows can reduce the weakly bound fraction.
- MEPS is field portable for remote sampling with or without the use of automated equipment.
- MEPS is adaptable for other analytical techniques including immunoassay and off-line analysis by NMR, IR and other methods.
Solid-phase extraction (SPE) has revolutionized sample preparation. Variations on the technique offer enhanced recovery, greater speciation and reduced solvent and sample consumption over other techniques.
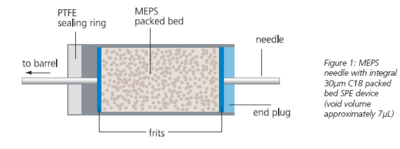
Micro-Extraction Packed Sorbent (MEPS) is the miniaturization of conventional SPE from milliliter to microliter bed volumes that allows SPE to be used with very small samples. The manipulation of the small volumes is achieved with a precision gas tight syringe. With a typical void volume of 7μL, the MEPS
elution is compatible with GC and LC inlets making it ideal for integration into an automated sampling system for on-line SPE. In most cases, MEPS allows the same level of sample concentration as is
possible with off-line conventional SPE while providing opportunities for truly hybrid multi-dimensional methods. MEPS methods may be readily adapted from established SPE methods including those based on mixed mode or complex chemistries.
Like SPE, MEPS is for use with liquid samples (either normal or reversed phase) and yields four fractions: the unretained, weakly bound, strongly bound and irreversibly bound. However, because MEPS is a double pass system (sample and solvent enter and exit from the bottom of the bed, the weakly bound fraction (commonly the interferences eliminated by washing) is less strongly bound. The irreversibly bound fraction affects MEPS and conventional SPE and is usually associated with sorbent wetting rather than sample purification
and so the irreversible binding of matrix material from one sample does not preclude reuse of the device for a sample of the same type.
Like conventional SPE, the number of times the device can be reused is
dependent on the sample matrix. For simple applications, MEPS devices have
been used successfully for >50 cycles.
Available for both GC & LC applications. There are over 65 pre-set methods.
Significant Markets
- Natural Products
- Environmental
- Food & Beverage
- Pharmaceuticals
Sample Methods
- Aflatoxin B2 and M2 Metabolite
- Amphetamine
- Catecholamines
- Cyclosporin
- F-2 Mycotoxin
- Opiate Anagelesics
- Phthalate Esters
- Prostaglandins
- Persistent Organic Pollutants (PAH, PCB, and Pesticides)
- Steroid Acids
- S-triazine Herbicide (Atrazine)
- Vitamin A, D, and E
Photos
Publications
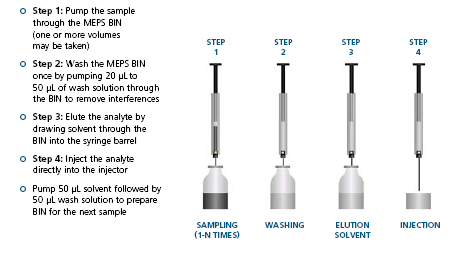
![]() Information specific to MEPS for CTC Autosamplers MEPS Online – The SPE solution for your CTC platform
Information specific to MEPS for CTC Autosamplers MEPS Online – The SPE solution for your CTC platform
MEPS Applications
![]() APPLICATION OF MEPS COUPLED WITH GAS CHROMATOGRAPHY TIME-OF-FLIGHT MASS SPECTROMETRY (GC-TOF MS) IN ANALYSIS OF BROMINATED FLAME RETARDANTS IN WASTE WATER
APPLICATION OF MEPS COUPLED WITH GAS CHROMATOGRAPHY TIME-OF-FLIGHT MASS SPECTROMETRY (GC-TOF MS) IN ANALYSIS OF BROMINATED FLAME RETARDANTS IN WASTE WATER
![]() Application to Opium Contaminated Animal Feeds
Application to Opium Contaminated Animal Feeds
![]() ON-SITE SAMPLE PREPARATION USING MEPS FOR WASTE WATER ANALYSIS
ON-SITE SAMPLE PREPARATION USING MEPS FOR WASTE WATER ANALYSIS
![]() FALSE POSITIVE DRUG TESTS FROM CONTAMINATED FEED
FALSE POSITIVE DRUG TESTS FROM CONTAMINATED FEED
![]() ANALYSIS OF BROMINATED FLAME RETARDANTS IN WASTE WATER
ANALYSIS OF BROMINATED FLAME RETARDANTS IN WASTE WATER
More Info
![]() MEPS web site by SGE including product brochure
MEPS web site by SGE including product brochure
![]() The Column article "It's a Small World"
The Column article "It's a Small World"
![]() The Column article "On-Site Sample Preparation Using MEPS for Wastewater Analysis
The Column article "On-Site Sample Preparation Using MEPS for Wastewater Analysis
MEPS Online Kit Application Index
ALKALOIDS
- A01 3,4-Diaminopyridine from Urine (Lambert-Eaton)
- A02 Alkylbenzyl Dimethylammonium Chlorides from Plasma
- A03 Amino Acids from Urine
- A04 Basic Nucleosides from Aqueous Solution
- A05 Bio-fl avonoids from Red Wine
- A06 Casuarine in Plant Extract
- A07 Catecholamines from Urine
- A08 Cyclodextrins from Plasma
- A09 Cyclodextrins from Urine
- A10 Dipterex in Serum
- A11 Diterpene Glycosides from Tea Extract
- A12 Fatty Acids from Serum
- A13 Homovanillic Acid from Plasma
- A14 Isofl avones from Plant Extract
- A15 Mixed Aromatic Amines from Urine and Plasma
- A16 Nicotine and Cotinine from Plasma
- A17 Nucleosides from Aqueous Solution
- A18 Nucleosides from Plasma
- A19 Nucleosides from Urine
- A20 PEG 400 in Serum
- A21 Persistent Organic Pollutants PAH, PCB and Pesticides in Blood
- A22 Persistent Organic Pollutants PAH, PCB and Pesticides in Plasma
- A23 Persistent Organic Pollutants PAH, PCB and Pesticides in Serum
- A24 Pesticides and PCB in Fats
- A25 Phenylanaline from Plasma
- A26 Prostaglandins from Urine
- A27 Prostaglandins from Whole Blood
- A28 Salsoline from Plasma
- A29 Steroid Acids from Serum
- A30 Tryptophan from Plasma
- A31 Vanillymandelic Acid from Plasma
- A32 Xanthines (Caffeine) from Serum
- A33 Xanthines (Theophylline) from Serum
ENVIRONMENTAL
- E01 Carbamate Insecticide (Aldicarb) from Water
- E02 PAH and PCB in Contaminated Soi
- E03 Phenols in water
- E04 Phthalate Esters in Water
- E05 S-triazine Herbicide (Atrazine) in Soil
FOOD & BEVERAGE
- F01 Afl atoxin B2 and M2 Metabolite Trace Analysis in Milk
- F02 Chloroacetanilide Herbicides (Acetochlor and Metolachlor )in Contaminated Water
- F03 F-2 Mycrotoxin Trace Analysis in Cereal
- F04 Fatty Acid Methyl Esters (Long Chain) in Fermentation medium
- F05 Omega 6 Fatty Acid in Malt Lipid Fractions
- F06 Pigment Anthocyanidins in Wine
- F07 S-triazine Herbicide (Atrazine) in Cereal
- F08 Sulfonamide Trace Analysis in Meat
PHARMACEUTICALS
- P01 Acetazolamide and Bumetanide in Urine
- P02 Acetazolamide in plasma
- P03 Amiodarone, Fendiline and Procainamide in Serum
- P04 Amphetamine in Plasma
- P05 Analgesics in Serum; Paracetamol and Tramadol
- P06 Anesthetics in Serum; Benzocaine, Mepivacaine, Procaineand Lidocaine
- P07 Antidepressants (Trycyclics) in Blood
- P08 Antidepressants (Trycyclics) in Urine
- P09 Atenolol in Plasma
- P10 Barbiturates in Serum; Barbital, Amobarbital, Phenobarbital,Secobarbital
- P11 Barbiturates in Urine; Barbital, Amobarbital, Phenobarbital,Secobarbital
- P12 Carbamazepine, Phenobarbital and Primidone in Serum
- P13 Chloramphenicol in Eye Drops
- P14 Cimetidine in Plasma
- P15 Cyclosporin in Blood
- P16 Erythromycin and Clarithromycin in Urine
- P17 Minor Tranquilizers (Benzodiazepines) in Urine or Serum
- P18 Minor Tranquilizers (Diazepam and Lorazepam) in Hair
- P19 Opiate Analgesics in Blood; Morphine and Codeine
- P20 Propranolol in Serum
- P21 Stobadin from Serum
- P22 Vitamin A, D and E in Supplements
- P23 Vitamin D3 and Metabolites in Serum
On-Line and Off-Line Application Of Micro-SPE (MEPS)
by Peter Dawes, Ern Dawes, Paul Wynne, SGE Analytical Science, and Dan DiFeo, SGE Inc.
Introduction Solid-phase extraction (SPE) has revolutionized sample preparation. Variations on the technique offer enhanced recovery, greater speciation and reduced solvent and sample consumption over other techniques. Micro-Extraction Packed Sorbent (MEPS) is the miniaturization of conventional SPE from milliliter to microliter bed volumes that allows SPE to be used with very small samples. The manipulation of the small volumes is achieved with a precision gas tight syringe. With a typical void volume of 7 L, the MEPS elution is compatible with GC and LC inlets making it ideal for integration into an automated sampling system for online SPE.
In most cases, MEPS allows the same level of sample concentration as is possible with off-line conventional SPE while providing opportunities for truly hybrid multi-dimensional methods. MEPS methods may be readily adapted from established SPE methods including those based on mixed mode or complex chemistries.
Figure 1. MEPS needle with integral 45-m C18 packed bed SPE device (void volume approximately 7 L). Click to enlarge Like SPE, MEPS is for use with liquid samples (either normal or reversed phase) and yields four fractions: the unretained, weakly bound, strongly bound and irreversibly bound. However, because MEPS is a double pass system (sample and solvent enter and exit from the bottom of the bed), the weakly bound fraction (commonly the interferences eliminated by washing) is less strongly bound. The irreversibly bound fraction affects MEPS and conventional SPE and is usually associated with sorbent wetting rather than sample purification and so the irreversible binding of matrix material from one sample does not preclude reuse of the device for a sample of the same type.
Like conventional SPE, the number of times the device can be reused is dependent on the sample matrix. For simple applications, MEPS devices
have been used successfully for 50 cycles.
Benefits of MEPS:
MEPS allows SPE methodology to be applied to small sample volumes. MEPS can be integrated into autosampler robotics and allows online use of SPE. MEPS can reduce sample and reagent consumption and waste disposal. Double pass flows can reduce the weakly bound fraction. MEPS is field portable for remote sampling with or without the use of automated equipment. MEPS is adaptable for other analytical techniques including immunoassay and off-line analysis by NMR, IR and other methods.
Plant Extracts Figure 2. Phytolacca octandra. The plant Phytolacca octandra (Figure 2) is one of the inkweeds or Pokes. In this application, the aerial portions of the plant were homogenized in acidified methanol, allowed to percolate for 12 hours, filtered and then extracted using a C2 MEPS cartridge. The MEPS was conditioned with methanol (30 L), water (30 L) and then 100 L of the plant extract was passed through the sorbent at 5mL/sec. The exhausted fraction was ejected at the same rate and the sorbent washed with 100mL of water. The sorbent was dried with air (3 80 L at 50 mL/sec) and eluted sequentially with hexane (10 L), dichloromethane (10 L) and methanol (10 L). The eluates were analyzed directly by GCMS on a BPX5 column (Figure 3).
The C2 MEPS method allowed the one step isolation of the FAME fraction (hexane) and the elimination of the highly polar sugar fraction (see Figure 3). Speciation of polar and non-polar analytes from a single sample digest was readily achieved without the need for off-line sample preparation.
Waste Water To demonstrate the usefulness of MEPS for dilute samples with a relatively simple matrix, a surrogate waste water sample was prepared from clear phenol free waste water spiked with either 25-ppb or 250-ppt phenols. The water was extracted with a C18 MEPS cartridge conditioned with methanol (30 L), water (30 L) and then 10 100 L of the water at 5 L/sec. The exhausted water was ejected at the same rate after each cycle; the sorbent was air dried (3 80 L at 50 L/sec). The analytes were eluted with methanol (10 L) and the fraction analyzed directly by GCMS on a BPX5 column. Chromatograms for the water samples are shown in Figures 4 and 5. Carryover was examined following the extraction of the 25-ppb sample by elution of a second and third portion of methanol without any intervening wash steps (Figure 5).
Figure 3. Effectiveness of the water wash in a GCMS analysis of Phytolacca octandra extract before and after fractionation on MEPS C2. Click to enlarge. Figure 4. Phenols in waste water at 250 ppt. Click to enlarge. Figure 5. Phenols in waste water at 25 ppb. Click to enlarge.
The C18 MEPS method allowed the one step isolation of phenols from water with good recovery, linearity and little carryover. The sorbent was reusable for the application for a large number of samples with no loss of performance after 10 analyses.
Toxicology Samples Papaver somniferum (opium poppy) is a feed contaminant that can result in positive drug tests for racing horses. We describe the extraction of a urine sample from an animal receiving contaminated feed as a demonstration of the off-line application of mixed mode C8/SCX MEPS for complex biological fluids.
Figure 6. Opiate metabolites in urine following MEPS of hydrolyzed urine. Click to enlarge. Here, 300 L of a diluted equine urine was hydrolyzed with b-glucuronidase or acid, filtered and extracted on a C8/SCX MEPS cartridge conditioned with methanol (30 L), potassium phosphate buffer (0.2 M, pH 6, 30 L) at a flow rate of 5 L/sec. The exhausted fraction was ejected at the same rate and the sorbent washed with 100-L phosphate buffer, 50-L acetic acid (1% v/v) and 100-L methanol. The sorbent was dried with air (3 80 L at 50 L/sec) and the sorbent eluted with 20-L dichloromethane-isopropanol-ammonia (49:49:2). The organic phase was evaporated under nitrogen and derivatized with 10 L of acetic anhydride-pyridine (1:2) at 80 C for 30 minutes before evaporation and reconstituted in 5 L of ethyl acetate. The extract was analyzed by GCMS on a BPX5 column (Figure 6).
The C8/SCX MEPS method allowed the microscale preparation of a small volume sample with comparable performance to conventional SPE techniques. Used off-line with derivatization and GCMS here, the sample was also suitable for online ESI-LCMSMS analysis by changing the elution solvent to methanol-ammonia (98:2) or methanol-trimethylamine (98:2).
For more information, contact Dan DiFeo, technical support, SGE Inc., at ddifeo@sge.com or by phone at 800-945-6154. Company’s Other Products Laboratory Equipment Rockaway NJ 07866
LEAP provides automated workstation instrumentation solutions based on the LEAP CTC PAL X, Y, Z syringe only autosampler robot from LEAP Technologies. This extremely flexible, precise, and adaptable liquid handling robotic platform is available in a variety of lengths and options depending on the requirements of your sample preparation and injections for your UHPLC, LC or GC chromatography.LEAP offers full support and service for the PAL platform in addition to being able to write custom macros, cycles, and scheduling to your applications. Please contact LEAP Technologies on how we can help you get maximized throughput with flexible pipetting automation solutions.
Contact LEAP
For additional information about LEAP and the PAL Platform, please contact LEAP Technologies. |