Hydrogen Deuterium Exchange
From LEAP
| Line 2: | Line 2: | ||
|name = Hydrogen Deuterium Exchange | |name = Hydrogen Deuterium Exchange | ||
|image = HDX_System.png | |image = HDX_System.png | ||
| - | |type = | + | |type = '''SPECIAL''' |
|id = HD-X PAL | |id = HD-X PAL | ||
|description = Automated Sample Prep for HD-X | |description = Automated Sample Prep for HD-X | ||
| Line 15: | Line 15: | ||
* Academia - Protein structural studies | * Academia - Protein structural studies | ||
| - | === | + | === Protein Structure === |
Protein function is strongly related to their tertiary structure and conformation. Chemical interactions which change this conformation can significantly affect protein functionality. This is important in studying disease states or effectiveness of drug candidates. | Protein function is strongly related to their tertiary structure and conformation. Chemical interactions which change this conformation can significantly affect protein functionality. This is important in studying disease states or effectiveness of drug candidates. | ||
Revision as of 15:55, 4 February 2009

| Hydrogen Deuterium Exchange |
| Application Type | |
| SPECIAL | |
| Application ID | |
| HD-X PAL | |
| Description | |
| Automated Sample Prep for HD-X |
Contents |
Overview
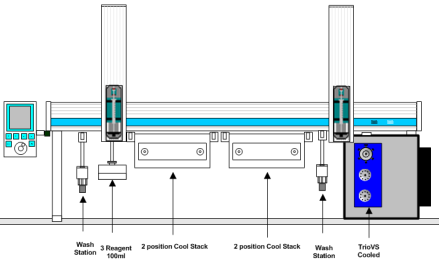
H/D-X PAL™ is an easy-to-use, system that provides an automated process for the scheduling and experimental execution of H/D-X experimental workflow. By use of the advanced LEAP Shell scheduling software experimental design is simplified and reliable. Synchronous reagent addition and sample labeling steps are automatically scheduled to increase throughput and produce high quality data.
Significant Markets
- Pharmaceutical - Drug Discovery,
- Academia - Protein structural studies
Protein Structure
Protein function is strongly related to their tertiary structure and conformation. Chemical interactions which change this conformation can significantly affect protein functionality. This is important in studying disease states or effectiveness of drug candidates.
There are 3 commonly used techniques to study protein structure:
1. Crystallography
2. NMR
3. HD Exchange
HD Exchange is a good approach in cases where there is insufficient sample (NMR) or the protein will not crystallize. It relies on the accurate measurement of the degree of labeling of a protein by deuterated hydrogen during a precisely measured labeling interval. The deuterium is exchanged with hydrogen atoms on the exposed amide backbone of the protein and depending on the which regions are exposed and how the protein is folded, differences are seen in the MS spectra of the digested protein when compared with non-labeled proteins.
Labeling time is important (exposure time of the protein to Deuterium) as is prevention of "back-exchange" after the labeling has occurred. This is the natural tendency of normal hydrogen to replace the deuterium after the label is removed. This is minimized by reducing the temperature of the mixture to close to zero degrees Celsius.
What is an HD-X experiment ?
Performing HD-X experiments manually involves setting up many labeling mixtures then quenching these mixtures at precisely timed intervals (by changing the pH) and then injecting them onto a chilled injection valve to the LCMS. In most cases a range of label times is used, from 10 seconds to several hours. Sources of error (apart from normal liquid handling inaccuracies) are timing errors, and degradation of sample due to "back-exchange". All of these sources of error are eliminated using the automated system described here.
What does the H/D-X PAL do?
The instrument has two cooled zones for sample storage and labeling. The injection valve is chilled to 1 degree Celsius and includes a solvent pre-chiller and column selection valve for in-line protein digestion.
A typical experiment would consist of around a dozen time points for each protein sample, ranging from 10 seconds to 8 hours. The PAL system will schedule the experiments and perform each of the following 3 steps for each time point and sample:
Labeling The protein is mixed with a Deuterium reagent and incubated for a precisely timed interval at room temperature.
Quenching The labeling reaction is stopped by transferring a volume of the mixture to an excess of quenching reagent in a pre-cooled vessel.
Injection A measured portion of the quenched reactants is injected into a multi-column, multi-valve LCMS system. This step is synchronized with the analytical system using hardware signals. The PAL will inject samples as the LCMS system becomes ready. Labeled and quenched reactants are held in the cooled stack until they have been injected and analyzed.
Software Control
LEAP has developed a scheduling application using their proprietary software “LEAP Shell” which will automate the above labeling steps, as well as the injection to the MS and the associated chromatography.
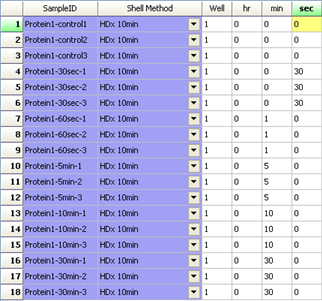
LEAP Shell Screen Shot 1: User interface to set up the labelling experiment:
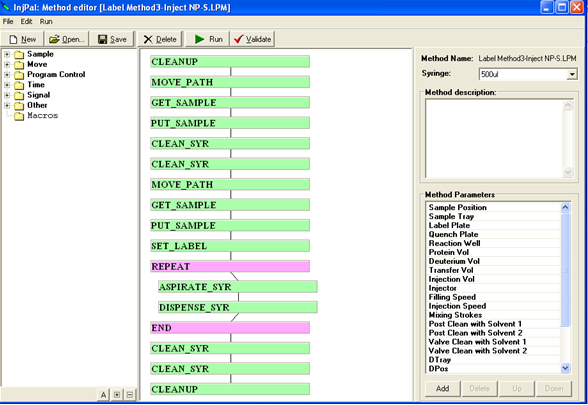
LEAP Shell Screen Shot 2: Powerfull editor for the PAL Methods:
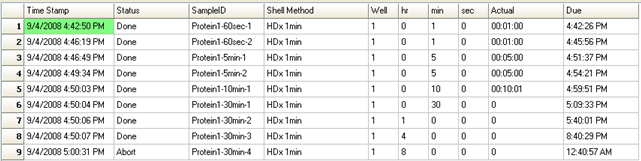
LEAP Shell Screen Shot 3: Run-time display of the labelling experiment with actual times:
Useful links
HXMS.com A good general reference site on HD Exchange theory and practice [1]
ASMS Interest Group - Hydrogen Exchange and Covalent Labeling [2]
LEAP Website H/D-x product description [3]
Publications