Forced Degradation - Dual PAL under LEAP Shell
From LEAP
Forced degradation is an important method used in drug development in the pharmaceutical industry. Forced degradation studies are used for multiple purposes, including demonstration of the specificity of separation methods, gaining insight into degradation pathways, and discernment of degradation products in formulations that are related to drug substances versus those that are related to other ingredients of a formulation. Reliable chemical stability testing data can show how a drug product changes over time with influence of environmental factors.
However, FDA guidance for forced degradation is vague with respect to experimental conditions. In order to harmonize the procedures of forced degradation, an automated method for forced degradation was developed, utilizing the CTC LEAP PAL workstation automation system. The Automated Forced Degradation approach significantly reduces the amount of manual labor used to perform the tests and harmonizes the operational procedures of forced degradation. The Automated Forced Degradation system is user-friendly and is intended to be used as a "walk-up system" that is able to prepare forced degradation and linearity samples, perform on-line HPLC analysis as well as generate reports automatically.
General Description of the Application
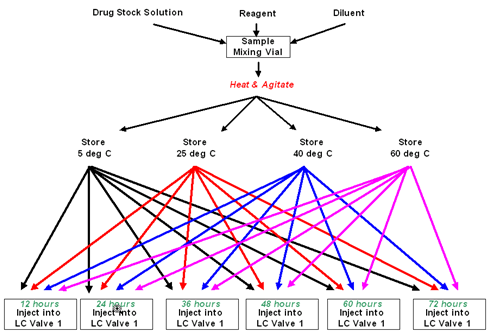
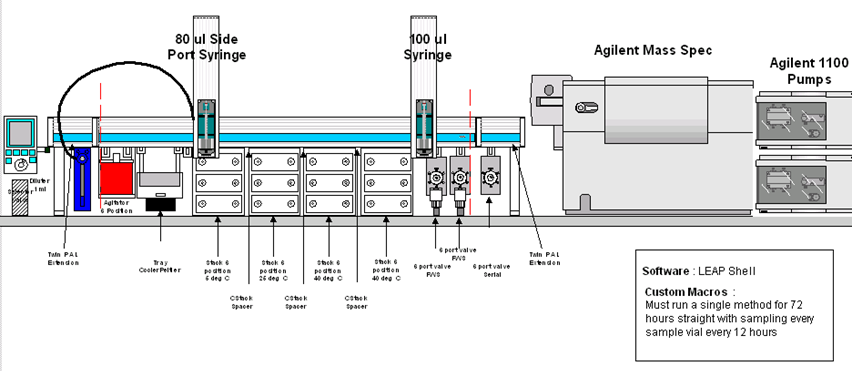
It is possible with the configuration of the PAL to automate accelerated degradation experiments. The Dual
headed PAL performs reaction mixture assembly followed by incubation of the samples at up to 4 different
temperatures. After specified times, over a period of hours or days, the PAL can then inject the reaction mixtures
directly onto the HPLC system. Stock solutions of drug and reagents are preloaded by the operator onto the PAL cooled tray (5 degrees Celsius). 4 other solvents are set up for dispensing using the large volume dilutor syringe. The PAL assembles the reaction mixtures according to the method parameters, transporting vials to a mixing station for a reaction and mixing period (e.g. 10 minutes at 50 degrees Celsius). Once the reaction is started in this way, aliquots of the mixture are transferred to microtiter plates in each of the 4 temperature controlled incubation stacks. Temperatures of each stack are controllable between 4 degrees Celsius and 40 degrees Celsius*.
After predetermined incubation times, small volumes of the samples will be aspirated from each of the samples batches at each temperature and injected into the HPLC system for analysis.
Project Ref: LS-T1006
Goals of the Automated System
- User friendly format – a “Walk-Up System”
- Reproducibly and accurately performs method validation and forced degradation
- Adaptability of system to changing conditions (solvents, degradation conditions, etc.)
LEAP strives to find total solutions for analytical lab automation by automating analytical processes for small and large molecules in extracted liquids, solids, and recently in human, animal and plant tissues. We provide the precise robotics and efficient sample prep required by modern measurement techniques such as MALDITOF mass spectroscopy. Our newest specialty customization of CTC Analytics’ PAL features small workstations that can perform complex liquid handling tasks including HPLC-Purification, SPE, filtration, weighing, heating and stirring. They can be configured as stand alone units or integrated for “just in time” sample prep for LC-MS or GC-MS analysis.
LEAP provides automated workstation instrumentation solutions based on the LEAP CTC PAL X, Y, Z syringe only autosampler robot from LEAP Technologies. This extremely flexible, precise, and adaptable liquid handling robotic platform is available in a variety of lengths and options depending on the requirements of your sample preparation and injections for your UHPLC, LC or GC chromatography.LEAP offers full support and service for the PAL platform in addition to being able to write custom macros, cycles, and scheduling to your applications. Please contact LEAP Technologies on how we can help you get maximized throughput with flexible pipetting automation solutions.
Contact LEAP
For additional information about LEAP and the PAL Platform, please contact LEAP Technologies. |