Forced Degradation - Dual PAL under LEAP Shell
From LEAP
Forced degradation is an important method used in drug development in the pharmaceutical industry. Forced degradation studies are used for multiple purposes, including demonstration of the specificity of separation methods, gaining insight into degradation pathways, and discernment of degradation products in formulations that are related to drug substances versus those that are related to other ingredients of a formulation. Reliable chemical stability testing data can show how a drug product changes over time with influence of environmental factors.
However, FDA guidance for forced degradation is vague with respect to experimental conditions. In order to harmonize the procedures of forced degradation, an automated method for forced degradation was developed, utilizing the CTC LEAP PAL workstation automation system. The Automated Forced Degradation approach significantly reduces the amount of manual labor used to perform the tests and harmonizes the operational procedures of forced degradation. The Automated Forced Degradation system is user-friendly and is intended to be used as a "walk-up system" that is able to prepare forced degradation and linearity samples, perform on-line HPLC analysis as well as generate reports automatically.
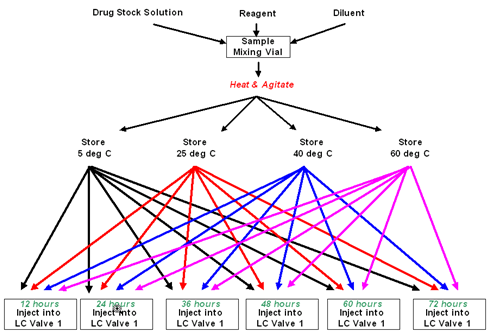
Reaction mixtures are prepared and then stress tested by incubating at up to 4 different temperatures. Over a period of time, samples are taken from each batch and injected to the HPLC to determine analyte stability.
Time intervals are determined in the Method. Batches can run from just a few hours to several days.
Experimental Workflow
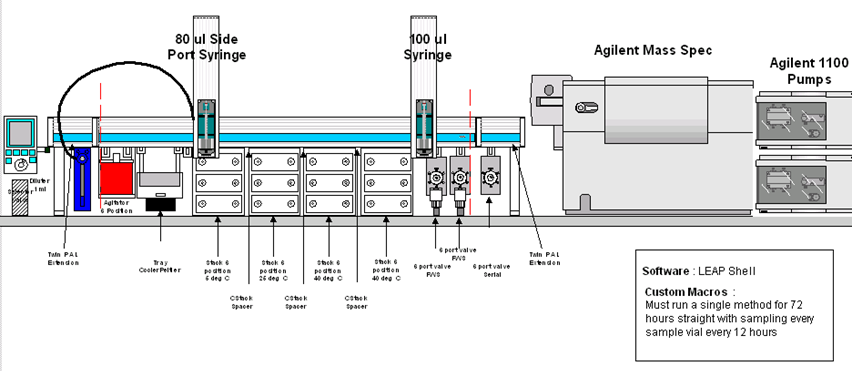
PAL System configuration
Project Ref: LS-T1006
LEAP strives to find total solutions for analytical lab automation by automating analytical processes for small and large molecules in extracted liquids, solids, and recently in human, animal and plant tissues. We provide the precise robotics and efficient sample prep required by modern measurement techniques such as MALDITOF mass spectroscopy. Our newest specialty customization of CTC Analytics’ PAL features small workstations that can perform complex liquid handling tasks including HPLC-Purification, SPE, filtration, weighing, heating and stirring. They can be configured as stand alone units or integrated for “just in time” sample prep for LC-MS or GC-MS analysis.
LEAP provides automated workstation instrumentation solutions based on the LEAP CTC PAL X, Y, Z syringe only autosampler robot from LEAP Technologies. This extremely flexible, precise, and adaptable liquid handling robotic platform is available in a variety of lengths and options depending on the requirements of your sample preparation and injections for your UHPLC, LC or GC chromatography.LEAP offers full support and service for the PAL platform in addition to being able to write custom macros, cycles, and scheduling to your applications. Please contact LEAP Technologies on how we can help you get maximized throughput with flexible pipetting automation solutions.
Contact LEAP
For additional information about LEAP and the PAL Platform, please contact LEAP Technologies. |