Forced Degradation - Dual PAL under LEAP Shell
From LEAP
Forced degradation is an important method used in drug development in the pharmaceutical industry. Forced degradation studies are used for multiple purposes, including demonstration of the specificity of separation methods, gaining insight into degradation pathways, and discernment of degradation products in formulations that are related to drug substances versus those that are related to other ingredients of a formulation.
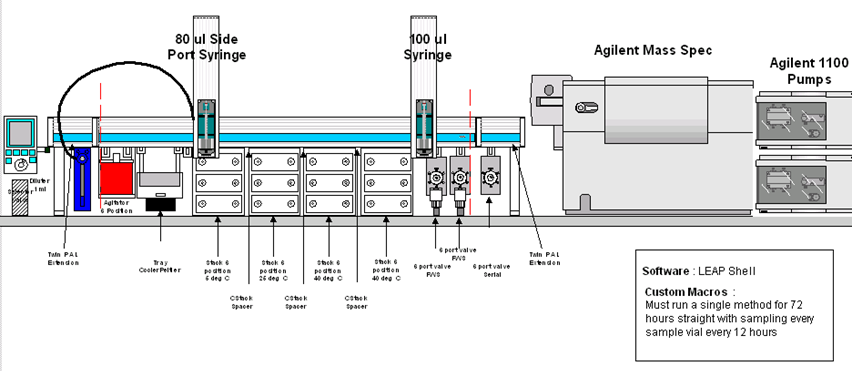
However, FDA guidance for forced degradation is vague with respect to experimental conditions. In order to harmonize the procedures of forced degradation, an automated method for forced degradation was developed, utilizing the CTC LEAP PAL workstation automation system. The Automated Forced Degradation approach significantly reduces the amount of manual labor used to perform the tests and harmonizes the operational procedures of forced degradation. The Automated Forced Degradation system is user-friendly and is intended to be used as a "walk-up system" that is able to prepare forced degradation and linearity samples, perform on-line HPLC analysis as well as generate reports automatically. The details of the system will be discussed along with a number of case studies demonstrating its use.
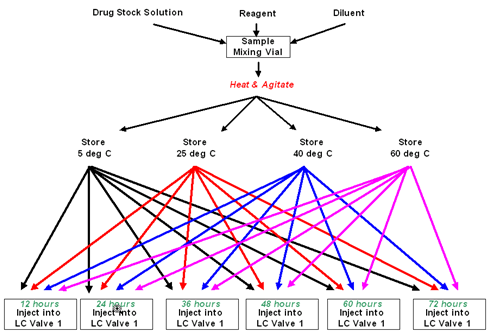
Reaction mixtures are prepared and then incubated at 4 different temperatures. Over a period of time, samples are taken from each batch and injected to the HPLC to determine analyte stability.
Time intervals are determined in the Method. Batches can run from just a few hours to several days.
Experimental Workflow
PAL System configuration
Project Ref: LS-T1006
Contact LEAP
For additional information about this technique please contact LEAP Technologies for detailed information